Basic Radiation Information
-
-
- For Further Details
What is radiation?
Radiation can be defined as small (subatomic) particles with kinetic energy that are radiated or transmitted through space.
One form of radiation possesses properties of light, with most of the radiation around us having this quality.
E.g.: X rays and gamma rays, which are used in medicineOther radiation possesses particle-like properties (so small, these particles cannot be seen with a microscope).
E.g.: alpha rays, beta rays (from radioactive materials), heavy particle radiation (used in treating cancer)
X rays and gamma rays are short wavelength
electromagnetic waves (with properties equivalent to light)All the various waves shown below are electromagnetic waves and related to light.
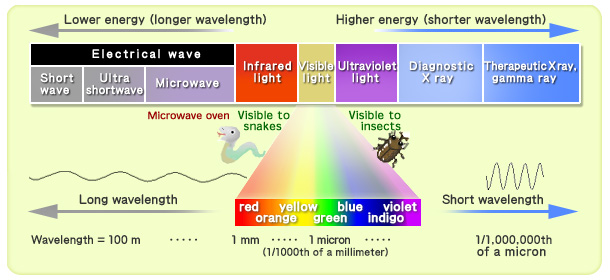
Electromagnetic waves (radiation) = flow of energy transmitted through space (Sohei Kondo)
When radiation’s wavelength is shorter than the wavelength of ultraviolet light (with increased energy), it can pass through the human body (as is the case with X rays and gamma rays). Even clothing cannot deter such radiation.
Alpha, beta, and gamma rays are radiation emitted from atomic nuclei, but X rays result when high-speed electrons collide with metal.
Neutron radiation is made up of particle rays and represents one aspect of A-bomb radiation.

How do radioactivity and radiation differ?
Radioactivity and radiation are different.
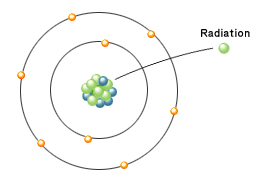
Radioactivity indicates the property of an unstable atomic nucleus changing (decaying) into a different atomic nucleus and emitting radiation…
In other words, decay of the atomic nucleus…
For that reason, the intensity of radioactivity is expressed as the number of atomic nuclei that decay per second. The intensity of radioactivity and the intensity of radiation are not the same.Decay of 1 nucleus per second = 1 becquerel
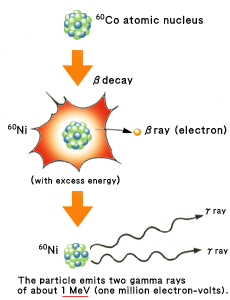
Model of decay of a 60Co atomic nucleus
Radiation also exists naturally
So long as humans live on Earth, we will always be exposed to small amounts of radiation.For people living at high elevations, there is an increase in exposure to cosmic radiation (radiation atop Mt. Fuji is five times higher than that at sea level).
When flying in an airplane roundtrip from Japan to New York City, passengers are exposed to about 0.1 mSv (millisievert) of radiation. The atmosphere surrounding Earth does have somewhat of a blocking effect on cosmic radiation.
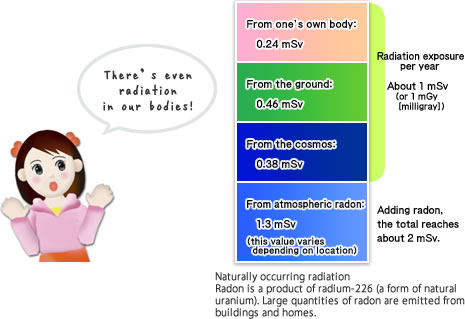
The radioactive element most prevalent in the human body is potassium-40 (40K). There is a total of about 130 grams of potassium in the body, with the radioactive form of potassium comprising 0.012% of that total. In other words, in our bodies each second 4,000 potassium nuclei decay, constantly emitting beta radiation. Apparently, most geothermal heat results from 40K (half life of 1.2 billion years)–and uranium also plays a role in generating this heat.
- For Further Details
-
