A Message from the Reticulocytes
Using reticulocytes, the immature red blood cells, RERF researchers have validated earlier findings that radiation exposure induces a dose-dependent increase in somatic gene mutations in humans.
by Seishi Kyoizumi and Mitoshi Akiyama, Department of Radiobiology, RERF
This article was originally published in RERF Update 6(3):5, 1994.
In late January of 1986, RERF’s Radiobiology Department was filled with the excitement of five scientists–Ron Jensen, then of Lawrence Livermore National Laboratory; Mike Bean, then a visiting scientist from Virginia Mason Research Center; RERF research scientist Nori Nakamura; and ourselves. We were plotting the glycophorin A (GPA) mutation data of atomic-bomb (A-bomb) survivors against tentative 1965 dosimetry (T65D) doses. Five months before–without providing information on radiation exposure, we had sent blood samples from 30 A-bomb survivors to Jensen and his coworkers, Rich Langlois and Bill Bigbee, who measured the GPA mutation frequencies of the survivors. Surprisingly, the plot in front of our eyes was showing a dose-dependent increase in mutations. Everybody in the room realized that this was the first evidence demonstrating radiation-induced somatic gene mutations in humans. In particular, Jensen was excited because the data indicated that erythrocyte variants detected using his assay were induced by genotoxic agents such as radiation. A year later, these results were published in Science (236:445-8, 1987).
Since then, the GPA assay has become widely accepted as a reliable way of measuring past radiation exposure. At RERF, we established an improved GPA assay for use in our large-scale studies of A-bomb survivors and other radiation-exposed persons (Cancer Res49:581-8, 1989).
Immature red blood cells provide RNA clues
However, one problem remained: we were not able to analyze mutated genes because erythrocytes lack nuclei. Therefore, the possibility that GPA-negative erythrocytes had been artifactually generated by antibody-staining errors lingered. During the intervening 7 years, biotechnology had advanced considerably, and polymerase chain reaction (PCR) made it possible to amplify DNA or RNA from a small amount of blood. Finally, we struck upon the idea of using reticulocytes, which are known to circulate in peripheral blood at a frequency of about 1% of total red cells and to contain various amounts of messenger RNA (mRNA). We suspected that a faint GPA “message” left in the reticulocytes could be amplified using PCR.
Because erythrocytes are replenished every 120 days, the origin of the radiation-induced GPA mutations in A-bomb survivors has to be the pluripotent stem cells from the bone marrow. Mature mutant erythrocytes differentiate from mutant CD34+ erythroid-committed progenitor cells (BFU-E) during the erythroblast and reticulocyte stages. Because expression of GPA molecules on the cell surface begins during the earlier erythroblast stage, we theorized that detection of later developing mutant reticulocytes would be possible.
To analyze GPA mutations in reticulocytes, we enriched the reticulocytes found in the peripheral blood using Percoll density gradient centrifugation. Afterwards, we confirmed by flow cytometry that 60%-70% of the cells in the low-density fractions consisted of reticulocytes expressing various amounts of mRNA.
As presented at the RERF Scientific Council meeting this year, we have developed a reverse transcription-polymerase chain reaction (RT-PCR) to detect GPA mRNA in reticulocytes. By using a two-step RT-PCR method, we can detect GPA mRNA extracted from as few as 100 reticulocytes from adult peripheral blood.
For preliminary analysis using this system, we analyzed Percoll-enriched reticulocytes from an A-bomb survivor who has an extremely high N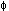 mutant frequency (about 3000 × 10-6). We sorted normal and N
mutant frequency (about 3000 × 10-6). We sorted normal and N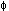 mutant reticulocytes from this survivor. Using RT-PCR, normal reticulocytes were shown to express both N- and M-type GPA mRNA by restriction-fragment-length-polymorphism (RFLP) analysis. In contrast, N
mutant reticulocytes from this survivor. Using RT-PCR, normal reticulocytes were shown to express both N- and M-type GPA mRNA by restriction-fragment-length-polymorphism (RFLP) analysis. In contrast, N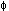 mutant reticulocytes express only N-type GPA mRNA and lack M-type GPA mRNA.
mutant reticulocytes express only N-type GPA mRNA and lack M-type GPA mRNA.
Thus, we demonstrated for the first time that GPA mutants detected by a cell sorter are really genetic mutants and not antibody-staining artifacts. Also, these data suggest that A-bomb radiation induced nonproductive-type mutations in the GPA gene–most probably gene deletion. To confirm this assumption, we will expand this reticulocyte study to other survivors having high mutant frequencies.
In vitro irradiation of erythroid progenitor cells
In a different approach, we are trying to induce GPA mutations in erythroblasts by means of in vitro irradiation of erythroid progenitor cells (BFU-E). Preliminary data suggest that mutant erythroblasts can be induced by x-irradiation. Because the two-step RT-PCR method can detect GPA transcripts produced in a single erythroblast, we can analyze the GPA message in radiation-induced mutant erythroblasts at the single-cell level. We will report the results of molecular analyses of mutant erythroblasts in the near future.
On the basis of the results of this experimental system, we might be able to compare the in vitro dose response with the survivors’ dose response, as well as characterize in greater detail the molecular nature of GPA mutations in erythroblasts using reticulocytes from the A-bomb survivors. These systematic approaches may clarify the properties of radiation-induced GPA gene mutations at the molecular level.

