Science, Radiation Protection, and the NCRP
The author discusses cancer risk estimates, which are based largely on the studies of atomic-bomb survivors, and their uncertainties.
by Warren K Sinclair, president emeritus, National Council on Radiation Protection and Measurements, Bethesda, Maryland
The following is an abridged version of the 17th Lauriston S Taylor lecture that was delivered in Washington on 7 April 1993 at the annual meeting of the National Council on Radiation Protection and Measurements (NCRP). This article originally appeared in RERF Update 6(2):3-5, 1994.
In this discussion, I will focus especially on uncertainties in current risk estimates for radiation-induced cancer. This risk is the largest component of health detriment due to radiation exposure at low doses and is estimated by the International Commission on Radiological Protection (ICRP) and NCRP to have a value, on average, of 5% per sievert for a population of all ages and 4% per sievert for an adult population of workers. These values are derived from the data in the RERF Life Span Study (LSS) of the atomic-bomb (A-bomb) survivors following high-dose-rate exposure using a dose and dose-rate effectiveness factor (DDREF) of 2, chosen by the ICRP and NCRP.
Uncertainties in risk estimates derived from the LSS can be grouped into five main categories: epidemiology, dosimetry, projection to lifetime, transfer between populations, and extrapolation to low dose and dose rate.
Epidemiological uncertainties
Epidemiological uncertainties include the statistical uncertainties associated with deriving the relatively few excess cancers (339) among a background of cancers resulting from all causes (5936), until 1985, in the 75,991 persons in the RERF Dosimetry System 1986 (DS86) sample. Also, since mortality data are based on death certificates, underreporting of deaths attributable to cancer, especially among older persons, gives rise to a significant error. The BEIR III committee used a factor of 1.23 to correct for this. But in its 1988 report, the United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR) made no correction, and, in its BEIR V report, the BEIR committee made no correction either.
Recently, researchers at RERF have examined the effect of diagnostic misclassification on noncancer and cancer mortality and have found that the excess relative risk for cancer should be increased by 13% for this reason (Sposto et al, Biometrics 48:605-17, 1992). The LSS sample, while representing a good cross section of all ages, may be somewhat unrepresentative in other ways, although probably less so than many clinical samples.
Dosimetrical uncertainties
Dosimetrical uncertainties include random and systematic errors in the dosimetry which have been estimated to be of the order of 25%-40%. Jablon (ABCC Technical Report 23-71), Gilbert et al (RERF Technical Report 12-82) and Pierce et al (Radiation Research 123:275-84, 1990) noted that these errors lead to bias errors in the risks, causing them to be smaller than the true risks in the higher dose range. This persistent bias in the dose-response curve would, if corrected for, require an increase in risk of 6%-17% for all dose points and 4%-11% if dose points above 4 Gy were eliminated.
The neutron component in DS86 is about 1%-2% of the absorbed organ dose in Hiroshima depending on organ site, distance, etc. Applying an average relative biological effectiveness (RBE) of 10 would cause the neutron equivalent dose to be about 10% of the total equivalent dose. Clearly, uncertainty is associated with this assignment.
The issue of the presence of “excess” thermal neutrons at distances in Hiroshima has caused much recent speculation (Preston et al, RERF Update 4[4]:5, 1993). If more neutrons were to be added at Hiroshima, agreement between the two cities on risk and on cytogenetic data would become distinctly poorer.
Projection to lifetime risk
Projecting from the observed population (39% of the total in 1985) to the lifetime of the entire population is one of the greatest potential uncertainties in our current estimates of lifetime risk.
The A-bomb survivor data for total cancer and for some individual organs, when classified by individual age groups, show that the excess relative risk (ERR) is approximately constant with time, even though a simple plot of ERR vs time for all age groups shows that the gross ERR for all solid cancers is still increasing.
In a different approach, Kellerer and Barclay (Radiat Prot Dos 41:273-81, 1992) suggested that attained age might be a better parameter than age at exposure for the dependence of sensitivity on age. Present data are complete only for the older ages in the population, and thus it is not possible yet to discriminate between these two models. If the attained-age model should ultimately prove correct, the presently used age-at-exposure model would overproject perhaps by as much as a factor of 2.
Uncertainty due to transfer between populations
Because of differing natural cancer rates among various populations in the world, it is difficult to know how to transfer risks from the exposed Japanese population to other populations. Transfer could be done either multiplicatively or additively.
In view of the uncertainties involved, the ICRP transferred the risk for individual organs by both the multiplicative and additive models and averaged the result, thus minimizing the potential error from this source. Fortunately, the ICRP average of five populations and the US estimate of the fractional organ risks do not differ greatly.
Uncertainty resulting from extrapolation to low dose rate
Many radiobiological phenomena involving low linear-energy-transfer radiations show reduced effectiveness for low dose rates as compared to high dose rates. In view of all these laboratory data (NCRP Report 64, 1980; UNSCEAR, 1993) and considering some less-definitive human information, the ICRP used an effectiveness DDREF of 2. NCRP would have preferred a larger value–perhaps 2 to 3 (and thus lower risk estimates), but accepted the ICRP value.
Dose response for solid tumors among the A-bomb survivors
A serious problem for choosing a value of the DDREF is that of the dose response for the solid-tumor data from the A-bomb survivors. For leukemia (which is about 10% of the total risk), the dose-response curve is best fit by a linear-quadratic expression that is compatible with a DDREF of 2 to 2.5. However, the other 90% of the risk is due to solid tumors that collectively are best fitted with a linear curve. This response can be stretched within the statistics of the data to include a DDREF of about 2 for mortality, but only to about 1.4 for the incidence data. Clearly, the linear fit over a dose range up to 4 Gy or 5 Gy is just that–a fit. It does not imply single-hit kinetics or any other mechanism. Furthermore, it is a composite of many different individual tumor responses. Again, a low-dose-rate response could actually be a linear curve of lower slope over the observed dose range. Nevertheless in the low-dose region, somewhere the high-dose-rate curve and the low-dose-rate curve must become the same, when there is less than one event per cellular target and no dose-rate effects can occur. Presumably this occurs at doses lower–possibly much lower–than present data provides.
I want to point out another way in which a lower slope (and thus a significant DDREF) can be reconciled with the apparent linear slope of the Japanese solid-tumor data. In some clearly defined circumstances, in-vitro cell killing at higher doses can account for a decreased incidence of transformation. Indeed, the slope of the decrement in transformation can be shown to be the same as that of a cell-killing curve. In the much more complex circumstances applying to intact tissues in vivo, such a simple influence of cell killing is not to be expected. Nevertheless, cell killing can be expected to play some role in limiting the number of viable cells at risk for cancer induction. Has cell killing been sufficiently considered in the assessment of the Japanese data? Here is one possible approach.
Let us suppose, simplistically, that the incidence of fatal cancer with dose is strictly a linear-quadratic I = 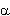 D +
D + 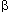 D2 and that what we observe is this incidence modified by a cell-killing term, K .f(D), which is a function of dose [ie, I = (
D2 and that what we observe is this incidence modified by a cell-killing term, K .f(D), which is a function of dose [ie, I = (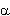 D +
D + 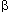 D2) K . f(D)]. Let us assume values for the parameters:
D2) K . f(D)]. Let us assume values for the parameters: 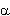 = 5% per sievert for the risk at low doses in a total population; a cross-over dose (ie,
= 5% per sievert for the risk at low doses in a total population; a cross-over dose (ie, 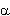 D =
D = 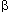 D2) of 1.2 Gy (a reasonable value); and thus
D2) of 1.2 Gy (a reasonable value); and thus 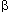 =
= 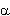 ÷ 1.2 or 0.83
÷ 1.2 or 0.83 . Then take for K . f(D) values from an experimental mammalian cell-survival curve for single cells in culture, eg, V79 cells exposed to cobalt-60 gamma rays from Hill et al (Radiation Research 113:278-88, 1988). The situation then is shown in Figure 1. This admittedly oversimplified and rather crude example shows that a lower initial slope than appears at first applicable could be possible, and it could account for a reasonably low value of DDREF, such as 2. The existence of a lower-than-linear initial slope in the low-dose region is further confirmed by a recent analysis by Shimizu et al (RERF Update 4[3]:3-4, 1992) of the region below 0.5 Sv. The relative risk for solid cancers is greater than 1 down to a dose of 0.02 Sv (ie, 20 mSv). Also, the analysis of data at less than 0.5 Sv leads to a linear slope somewhat lower than for the slope of the linear response for all the data up to 4 Sv. This is in concert with the linear-quadratic response and cell-killing notion described above.
. Then take for K . f(D) values from an experimental mammalian cell-survival curve for single cells in culture, eg, V79 cells exposed to cobalt-60 gamma rays from Hill et al (Radiation Research 113:278-88, 1988). The situation then is shown in Figure 1. This admittedly oversimplified and rather crude example shows that a lower initial slope than appears at first applicable could be possible, and it could account for a reasonably low value of DDREF, such as 2. The existence of a lower-than-linear initial slope in the low-dose region is further confirmed by a recent analysis by Shimizu et al (RERF Update 4[3]:3-4, 1992) of the region below 0.5 Sv. The relative risk for solid cancers is greater than 1 down to a dose of 0.02 Sv (ie, 20 mSv). Also, the analysis of data at less than 0.5 Sv leads to a linear slope somewhat lower than for the slope of the linear response for all the data up to 4 Sv. This is in concert with the linear-quadratic response and cell-killing notion described above.
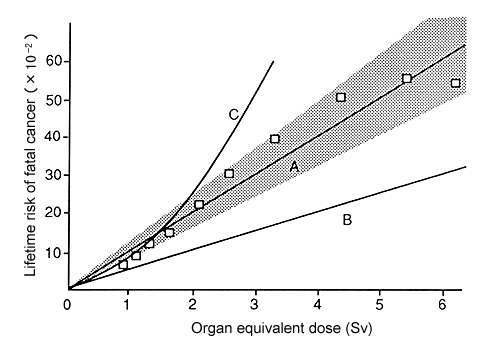
Figure 1. Curve A represents the “observed” high-dose-rate Japanese data line (slope 10 × 10-2Sv-1). Curve B represents the ICRP risk line obtained with a dose and dose-rate effectiveness factor (DDREF) of 2, viz, 5 × 10-2Sv-1. Curve C is the beginning of a curve of incidence vs D + 0.83D2, not corrected for cell killing. The set of points (squares) is for D + 0.83D2)K·f(D), ie, the linear quadratic with the experimental cell-killing values K·f(D). The set of points begins below curve A, fits well over a range, and then begins to level off. It could be reasonably fitted by the 10%-per-sievert line (see the 95% confidence limits on curve A), but its initial slope is only 5% per sievert, equivalent to a DDREF of 2; ie, within the limits of error, we could have an initial slope of only 5% per sievert, derived apparently from data that with a good linear fit yield 10% per sievert.
Does a threshold for induced cancer exist?
While it is true that in view of conflicting sources of information and statistical uncertainties, a threshold for induced cancer cannot be entirely ruled out, I nevertheless want to make a few points about the issue of a threshold. Although it is possible to imagine a line of lower slope than the 10% per sievert high-dose-rate line derived from the Japanese data as being the true situation for low dose rate, as we have shown above, a threshold is much more difficult to explain. It involves a discontinuity that requires, in my view, a new process operative only at low doses that somehow leads to the dotted portions of curves A and B, as shown in the expanded low-dose portion of Figure 2. It is not impossible to imagine such a process, and indeed something like it has been shown by S Wolff and colleagues for a transient repair effect for chromosome-aberration induction in human lymphocytes at low doses (Shadley et al, Radiation Research111:511-17, 1987). However, this phenomenon has not seemed either to last very long or to be generally applicable to, eg, the case of cancer induction.
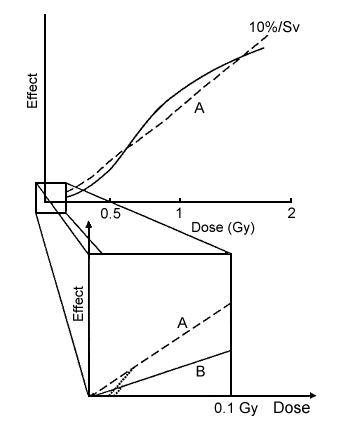
Figure 2. Effects vs dose, expanded in the low-dose region. Line A () is linear, line B () is linear quadratic, and the dotted lines represent possible response.
Some outstanding laboratory studies of the dose response in the low-dose region are noteworthy. One by Lloyd et al (International Journal of Radiation Biology 61:335-43, 1992) involves the study by six laboratories of chromosome aberrations in lymphocytes that found linearity in dose vs effect down to 20 mGy. Some studies in the past have extended to even lower doses, notably Sparrow et al (Science 176:916-18, 1972) with pink mutations in Tradescantia that extend to 2.5 mGy of x rays and 0.1 mGy of neutrons. When plotted on a linear scale, the x-ray data still show linearity. Also Bateman et al (Radiation Research 51:381-90, 1972) studied lens opacities after neutron doses as low as 0.22 mGy and found that controls differed significantly.
Thus, we may ask at what dose levels are thresholds expected to start? We seem to have measured linearity in some systems to about 2 mGy of x rays and to 1/10th of a milligray for neutrons. Is there any reason to suppose linearity will not continue to even lower doses? At very low doses and therefore very low risks, does it matter if a nominal threshold exists? Both the doses and the risks would be regarded by many as negligible. In my view, given the stochastic nature of the process, it is sounder to accept the nonthreshold hypothesis and describe the risk as negligible at very low doses than to argue for a threshold.
Low-dose epidemiological studies
Over the years, a multitude of low-dose epidemiological studies have raised many questions about radiation effects in humans but have individually contributed relatively little to risk estimation for a variety of reasons. However, recently low-dose studies on workers in the United Kingdom and also in Russia, as well as one environmental study in Russia, have contributed some new numbers. A comparison with the Hiroshima-Nagasaki-derived estimates indicate that the UK study has a higher leukemia risk, which is partially balanced by the negative result from a smaller US study. The two Russian studies, while based on rather uncertain data up to now, appear to be in concert with the derived high-dose values. More data of this type, even though it has very wide confidence intervals, would be extremely useful for radiation protection. A proposed study of all US workers at nuclear-power plants could be most valuable but has so far not been initiated. The scientific community and perhaps especially the NCRP should press to see that this takes place. Such a study and its potential incorporation in a larger worldwide study under the aegis of the International Agency for Research on Cancer (IARC), Lyon, could be a most important step for radiation protection. In the meantime, it seems that a less ambitious but also useful study will be done by IARC, combining the US, UK, French, and Canadian data.
Radiation protection recommendations
Given all the above, where does the NCRP stand with its current recommendations? Overall, I think the uncertainties are about as likely to lead to higher risk estimates as lower, see Table 1 (though I tend to favor them being lower, mainly because of the possibility of overprojection). In the future, I hope they lead to lower risk estimates because, in fact, the ICRP in 1990 (Publication 60) did not aim for a higher “level of ambition” for radiation protection than in 1977, ie, the relationship of the total detriment to the limits is about the same. NCRP went a little further with its guidance in 1987 (Report 91) and its limit in 1993 (Report 116). Furthermore, the NCRP base of comparison with worker risks is updated to the present. In any case, I would rather find that the present risk estimates are a little too high so that when they are next used as the basis of recommendations–perhaps in the early years of the new century–changes might not be needed because the risks would be in concert with other normal worker risks at that time.
|
Factors related to uncertainties
|
Approximate contribution
|
| In support of higher risk estimates | |
| +10% | |
| +13% | |
| +?% | |
| In support of lower risk estimates | |
| ? -(13-22)% | |
| ? -(25-50)% | |
| Of equal impact for higher or lower risk estimates | |
| ? 25-50% | |
| ? 50% | |
Thus, in my opinion, the ICRP in 1990 and the NCRP in 1987 and in 1993, even more so, have correctly lowered occupational and public limits in response to our best present information on risk estimates despite the many uncertainties in these estimates.

