Breast Cancer in the RERF Life Span Study
Dose-related excess risk may be profoundly influenced by genetic factors and reproductive history.
by Charles E Land, Radiation Epidemiology Branch, National Cancer Institute, Bethesda, Maryland, and M Tokunaga, Department of Epidemiologic Pathology, RERF
This article was originally published in RERF Update 5(1):3-4, 1993.
Increased breast-cancer risk in women is a relatively well quantified late effect of atomic-bomb radiation exposure. It has also been studied extensively among patients receiving multiple X-ray exposures for diagnostic or therapeutic purposes related to tuberculosis, scoliosis, benign breast disease, or enlarged thymus. Data from medically irradiated populations and the RERF Life Span Study (LSS) sample became available at about the same time and have developed more or less in parallel; the first study by C Wanebo et al (New Engl J Med 279:667-71, 1968) of breast-cancer incidence in the ABCC-RERF clinical subsample was prompted by I MacKenzie’s finding of increased risk among former patients of a Nova Scotian tuberculosis sanitorium (Br J Cancer 19:1-8, 1965).
The particular scientific value of the series of LSS sample studies is enhanced by two important facts: First, radiation-related risk among women exposed during childhood or adolescence is higher than risk following exposure during adult life, and the LSS sample, whose age distribution in 1945 was typical of urban Japan, has a large fraction of survivors exposed when young. Second, parallel analyses by J Boice et al (Radiology131:589-97, 1979), C Land et al (JNCI 65:353-76, 1980) and, most recently and convincingly, D Preston et al (in preparation), have made it clear that the risk of radiation-related breast cancer is about the same for comparable groups of exposed Japanese and Caucasian women, even though normal breast-cancer rates are far lower in Japan. Thus, at any given radiation dose, the ratio of excess risk to baseline risk is much greater in the LSS sample than in any other population now being studied, which means that the LSS data tend to be more informative about details of risk and about the nature of radiation-induced cancer.
The most recent site-specific study of breast cancer in the LSS sample (M Tokunaga et al, Radiat Res 138:209-223, 1994) is based on 807 cases diagnosed from 1950-85, including 68 survivors who were under 10 yr old at the time of the bombings (ATB) and 230 who were between 10 and 19 yr old ATB. As might be expected, findings with respect to dose response and its modification by age ATB, attained age, and time following exposure were similar to those reported by D Thompson et al (Radiat Res 137S:17-67, 1994) on the basis of tumor-registry diagnoses from 1958-87 for that part of the LSS sample still living in Hiroshima and Nagasaki. In particular, the dose response is marked and strongly linear, excess relative risk (ERR) decreases with increasing age ATB (Figure 1) or increasing attained age, and, controlling for age, ERR varies little by time following exposure. Risk was relatively low for women 40 yr old or older ATB, but there is now a statistically significant excess in that group.
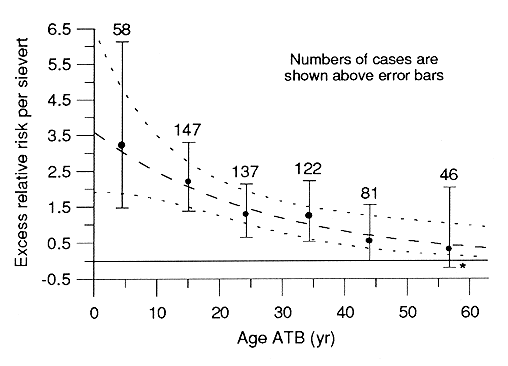
Figure 1. Fitted linear-model estimates of excess relative risk per sievert with 90% confidence limits by age at the time of the bombings (ATB) for discrete intervals and as a smooth function of age. *Minimum feasible value for lower limit.
A surprising finding from the more detailed analysis in the site-specific study is that the ERR was markedly higher for early-onset breast cancer (ie, cases diagnosed before age 35) than for cancer diagnosed at later ages. The contrast is extreme: among women under 20 yr old ATB, the ERR at 1 Sv was 13.5, with 90% confidence limits (4.4, 68.9) for early-onset breast cancer vs an ERR of 2.0 (1.3, 3.0) at ages 35 or older ATB; moreover, age-specific estimates were essentially uniform for attained ages 35-44, 45-54, and 55 yr or older (Figure 2). One possible explanation is that it may reflect the existence of a genetic subgroup with high sensitivity to radiation-induced breast cancer. This interpretation is supported by recent findings regarding the genetic basis for breast-cancer risk, especially at early ages: less than 1% of the general population is thought to account for one-third of the population risk before age 29, due to an inherited, autosomal dominant mutation (E Clauss et al, Am J Hum Genet 48:232-42,1991; B Newman et al,Proc Natl Acad Sci USA 85:3044-8, 1988). Because all the somatic cells of women with this inherited syndrome would already have one mutated allele, they might be unusually sensitive to radiation. It seems likely that a tumor-suppressor gene is involved, a possibility currently being investigated at RERF.
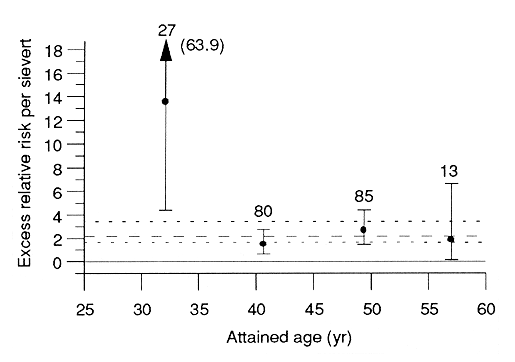
Figure 2. Fitted linear-model estimates of excess relative risk per sievert with 90% confidence limits for women under 20 yr old at the time of the bombingsbyinterval of attained age and for all attained ages combined. Numbers of cases are shown above error bars.
It is remarkable that, with the exception of a few early-onset cases, radiation-related breast-cancer risk closely tracks underlying age-specific population rates in the sense that, for fixed age ATB, ERR shows no trend with either attained age or time following exposure. This suggests that all breast cancers, whether or not radiation-related, are influenced similarly by other factors that vary with age. In all general populations studied, reproductive history is strongly related to risk. In particular, women with a first full-term pregnancy before age 20 have about one-third the risk of women who did not have such a pregnancy before age 30, and other factors related to that variable, such as number of children and lactation history, are also correlated with risk.
A case-control interview study, based on cancers diagnosed before 1980 and matched to controls on the basis of age ATB, city, and radiation dose, was carried out to determine (1) if the usual risk factors were operating in the LSS sample, (2) whether these factors interacted with radiation dose to influence breast-cancer risk, and (3) whether differences in radiation-related ERR by age ATB could be explained in terms of reproductive history ATB. As expected, age at first full-term pregnancy was strongly, and positively, related to risk (Figure 3), as were several correlated variables (C Land et al, Cancer Causes Control5:157-165, 1994). Number of births and total cumulative lactation period each were strongly, and negatively, related to risk even after adjustment for age at first full-term pregnancy.
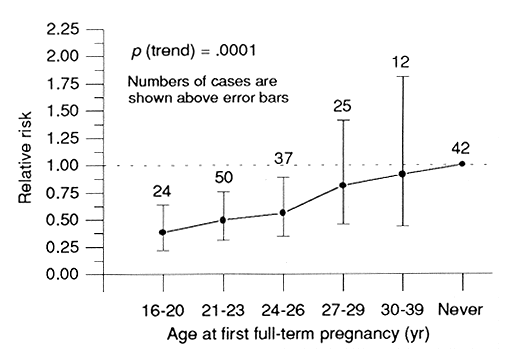
Figure 3. Estimated relative risk of breast cancer by age at first full-term pregnancy
(90% confidence intervals).
Interaction of the above factors with radiation dose was investigated using estimates of dose-related ERR (see Figure 1) obtained from incidence data (C Land et al, Cancer Causes Control 5:167-176, 1994). For all three of the factors, an additive relationship with dose could be ruled out at a high level of confidence, whereas a multiplicative (ie, synergistic) relationship was consistent with the observations (Figure 4). Thus, for example, early age at first full-term pregnancy appeared to be protective against both baseline and radiation-related breast-cancer risk in approximately the same ratio (ie, a threefold reduction of baseline cancer risk is accompanied by about the same reduction in dose-related risk at any dose level). The evidence for synergy was essentially limited to the first of the three factors, in the sense that the evidence against additivity for the other two appeared to be explainable in terms of their correlations with age at first full-term pregnancy.
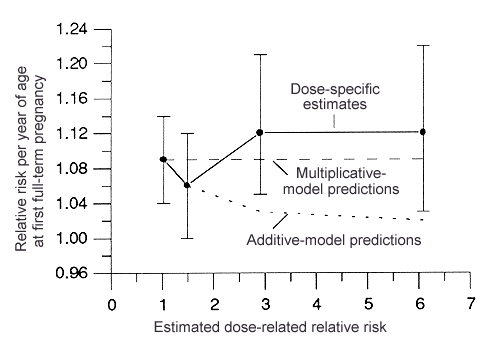
Figure 4. Estimated relative-risk multiplier per year of age at first full-term pregnancy: dose-specific values vs corresponding predictions, according to the multiplicative and additive models for interaction with radiation dose.
Analyses still in progress concern the role of reproductive history ATB as a modifier of radiation dose response. The relationship appears to be complex. One interesting preliminary result is that the apparently protective effect against radiation-induced breast cancer of early age at first full-term pregnancy seems clear among women who did not experience such a pregnancy before radiation exposure or among women exposed during childhood or adolescence (which is almost the same thing). For women exposed after their first full-term pregnancy or as adults, however, evidence for synergy is weaker, probably because the level of dose-related excess risk is lower.
To our surprise, earlier breast-cancer data from the LSS sample revealed that there is (1) a high radiation-related risk in this population, which has one of the world’s lowest breast-cancer rates and (2) an excess risk from exposure before breast development. Recently, we also have been surprised by evidence suggesting a strong genetic component to the risk of radiation-related, early-onset breast cancer and by the contrast between the additive interactive relationship of radiation dose with baseline risk (Japan vs the US) and the apparently synergistic relationship of dose with reproductive history. It seems likely that radiation competes with other risk factors related to certain differences in breast-cancer rates worldwide, but that the influence of reproductive history is expressed by promoting factors that act similarly upon potential cancers related to radiation exposure or other cancer initiators.
If present risk patterns continue, we can expect about 480 more breast cancers during the remaining lifetimes of the LSS members, of which about 155 may be related to radiation dose. More than 70% of the new cases and 90% of the radiation-related cases are expected to occur among women who were under 20 yr old ATB. Thus, we may expect more information from continued follow-up of this population.

