How Radiation Affects Cells
-
How radiation affects cells
Radiation
Ionizing radiation is energy transmitted via X rays, gamma rays, beta particles (high-speed electrons), alpha particles (the nucleus of the helium atom), neutrons, protons, and other heavy ions such as the nuclei of argon, nitrogen, carbon, and other elements. X rays and gamma rays are electromagnetic waves like light, but their energy is much higher than that of light (their wavelengths are much shorter). Ultraviolet (UV) light is a radiation of intermediate energy that can damage cells (the well known sunburn), but UV light differs from the forms of electromagnetic radiation mentioned above in that it does not cause ionization (loss of an electron) in atoms or molecules, but rather excitation (change in energy level of an electron). The other forms of radiation–particles–are either negatively charged (electrons), positively charged (protons, alpha rays, and other heavy ions), or electrically neutral (neutrons).
Ionization
As an example of ionization, beta rays are fast electrons that lose energy as they pass through cells and interact with molecules. The transferred energy is high enough to disrupt chemical bonds, which results in radical formation (or ionization). Ionization differs from the ion formation that occurs in ordinary chemical reactions. The process that takes place when salt (sodium chloride, NaCl) is dissolved in water is a good example of an ordinary reaction. Sodium and chloride bind together because, separately, each atom is unstable. The sodium (Na) atom has only one electron in its outermost orbit, and loss of that electron makes it more stable. In contrast, the chloride (Cl) atom has seven electrons in its outermost orbit and gaining one electron to have a full complement of eight outer electrons makes it more stable. When the two atoms bind to form NaCl, sodium shares its single outer electron with chloride, and so, both are stable. In ordinary chemical reactions, such as the binding of Na to Cl, electrons that are lost or gained are always those on the outermost orbit. When NaCl is dissolved in water, the two atoms separate, with chloride keeping the extra outer electron; thus, the sodium has a net positive charge (hence Na+) and the chloride has a net negative charge (hence Cl–), but the net charge (balance between positive and negative) remains neutral. These charged atoms are called ions, and they are stable in water despite their electrical charges.
In contrast, when an electron passes through a cell, it releases its energy along its path (called a track) by interacting with the electrons of nearby molecules. The released energy is absorbed by atoms near the track, resulting in either excitation (a shift in the orbit of an electron to a higher energy level) or ionization (release of an electron from the atom). What differs from an ordinary chemical reaction is that when radiation donates energy to atoms or molecules, electrons other than those on the most outer orbit can be released, which makes the atoms very unstable. Such unstable atoms are called radicals and are chemically very reactive. Some radicals are so reactive that they exist only for as short a time as a microsecond.
X and gamma rays differ from beta particles in that they release high-speed electrons from atoms first. Positively charged particles transfer energy to molecules in cells by essentially the same mechanisms. Neutrons are somewhat different since they are electrically uncharged, and their main effect is to impact the nuclei of hydrogen atoms, namely protons. Since the masses of a neutron and a proton are similar, the impact results in an elastic scattering process like in billiards. The ejected protons behave as charged particles.
How ionizations affect cells
Radiation-induced ionizations may act directly on the cellular component molecules or indirectly on water molecules, causing water-derived radicals. Radicals react with nearby molecules in a very short time, resulting in breakage of chemical bonds or oxidation (addition of oxygen atoms) of the affected molecules. The major effect in cells is DNA breaks. Since DNA consists of a pair of complementary double strands, breaks of either a single strand or both strands can occur. However, the latter is believed to be much more important biologically. Most single-strand breaks can be repaired normally thanks to the double-stranded nature of the DNA molecule (the two strands complement each other, so that an intact strand can serve as a template for repair of its damaged, opposite strand). In the case of double-strand breaks, however, repair is more difficult and erroneous rejoining of broken ends may occur. These so-called misrepairs result in induction of mutations, chromosome aberrations, or cell death.
Characteristics of DNA damage by radiation exposure
Deletion of DNA segments is the predominant form of radiation damage in cells that survive irradiation. It may be caused by (1) misrepair of two separate double-strand breaks in a DNA molecule with joining of the two outer ends and loss of the fragment between the breaks or (2) the process of cleaning (enzyme digestion of nucleotides–the component molecules of DNA) of the broken ends before rejoining to repair one double-strand break.
Biological effects differ by type of radiation
Radiations differ not only by their constituents (electrons, protons, neutrons, etc.) but also by their energy. Radiations that cause dense ionization along their track (such as neutrons) are called high-linear-energy-transfer (high-LET) radiation, a physical parameter to describe average energy released per unit length of the track. (See the accompanying figure.) Low-LET radiations produce ionizations only sparsely along their track and, hence, almost homogeneously within a cell. Radiation dose is the amount of energy per unit of biological material (e.g., number of ionizations per cell). Thus, high-LET radiations are more destructive to biological material than low-LET radiations–such as X and gamma rays–because at the same dose, the low-LET radiations induce the same number of radicals more sparsely within a cell, whereas the high-LET radiations–such as neutrons and alpha particles–transfer most of their energy to a small region of the cell. The localized DNA damage caused by dense ionizations from high-LET radiations is more difficult to repair than the diffuse DNA damage caused by the sparse ionizations from low-LET radiations.
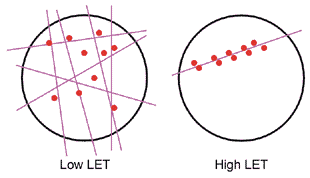
Figure. Both examples produce the same total number of ionizations, thus represent the same dose.

